News
bioMérieux, a world leader in the field of in vitro diagnostics, today releases its business review for the three months ended March 31th, 2024.

Swipe to discover more
-
bioMérieux presents its new 5-year strategic plan and its targets for profitable growth
bioMérieux, a world leader in the field in vitro diagnostics, is hosting today a Capital Markets Day in Paris. On this occasion, Pierre Boulud, Chief Executive Officer, together with members of the Executive Committee will present bioMérieux’s strategic plan, GO•28, the new chapter for the Company’s growth and development.
-
Rapid Diagnostics & Antimicrobial Stewardship Can Guide Treatment for Sepsis Patients: Interview with Dr. Eric R. Wenzler
As pathogens change over time, they can become resistant to antibiotics, making it more difficult to treat infections that may lead to sepsis. This ongoing growth of antimicrobial-resistant bacteria continues to pose a threat to public health. We sat down for a virtual interview with Eric R. Wenzler, PharmD, BCPS, BCIDP, AAHIVP, Assistant Professor in the Department of Pharmacy Practice at the University of Illinois at Chicago College of Pharmacy, to learn more about the role of diagnostics and stewardship intervention when caring for sepsis patients.
-
bioMérieux Named Business of the Year by Utah Governor’s Office of Economic Opportunity
bioMérieux, a world leader in the field of in vitro diagnostics, has received the 2024 Business of the Year Award, presented by the Utah Governor’s Office of Economic Opportunity at One Utah Summit April 11-12, in Salt Lake City.
Enabling Decision-Making
- ANTIMICROBIAL STEWARDSHIP
- SEPSIS
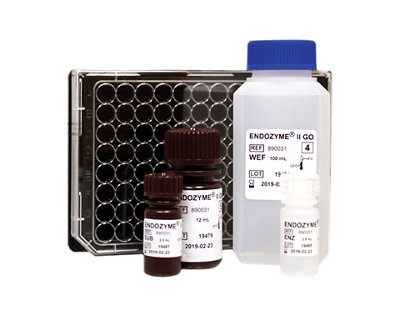
- FOOD SAFETY & QUALITY
- PHARMA QUALITY CONTROL
- COVID-19
- ANTIMICROBIAL STEWARDSHIP
- SEPSIS
- FOOD SAFETY & QUALITY
- PHARMA QUALITY CONTROL
- COVID-19











?qlt=85&ts=1670238196189&dpr=off)