ARGENE® Respiratory
R-GENE® Respiratory PCR Kits
ARGENE® Respiratory includes ready-to-use real-time PCR detection kits for viral respiratory diseases.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
- ARGENE Respiratory Range
- Overview
- Assays
- Resources
Overview
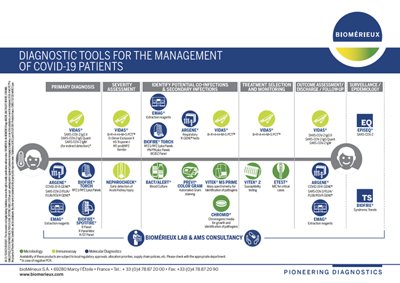
Targeted Diagnostic Strategy with an “A La Carte” Menu
Respiratory pathogens have variable seasonalities with some being present throughout the year and others present during epidemic outbreaks. Diagnostic strategies can be adapted to the season by searching for the most likely pathogens in 1st intention and the remaining pathogens in 2nd intention.
Everything You Need in One Kit
R-GENE® kits include all necessary reagents:
- Ready-to-use amplification premix (including Taq polymerase)
- Reverse transcriptase
- Positive control
- Negative control
Easy Procedure
Using R-GENE® assay is easy.

ARGENE® Expertise
- Simplicity: complete kits, ready-to-use reagents, same pipetting procedure
- Seamless Integration: validated for use on multi-specimens, multi-extraction, and multi-amplification platforms
- Lab Efficiency: common internal control, harmonized extraction and amplification protocols, multiple targeted detection from one extracted sample
Winter Respiratory Tract Infections Challenges
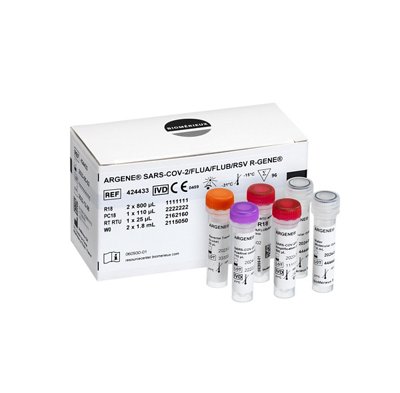
SARS-COV-2/FLUA/FLUB/RSV R-GENE®
Assays
| Kit Designation | Reference | Type of Kit | Number of Tests | Regulatory Status* |
|---|---|---|---|---|
| SARS-COV-2/FLUA/FLUB/RSV R-GENE® | 424433 | Real-time Detection Kit | 96 | For in vitro diagnostic use (IVDR) |
| COVID-19 R-GENE® | 424017 | Real-time Detection Kit | 96 | For in vitro diagnostic use (IVDR) |
| RHINO&EV/CC R-GENE® | 71-042 | Real-time Detection Kit | 60 | For in vitro diagnostic use (IVDR) |
| SARS-COV-2/FLUA/FLUB/RSV R-GENE® | |
|---|---|
| PCR DESIGN | 5-PLEX
|
| TARGETED GENES |
|
| CONTROLS INCLUDED | Positive Control, Negative Control, Endogenous Internal Control |
| SPECIMENS |
|
| EXTRACTION PLATFORMS |
|
| AMPLIFICATION PLATFORMS |
|
| RESULT WITHIN | 90 minutes after extraction |
| STORAGE CONDITIONS | -15°C / -31°C |
| STATUS | For in vitro diagnostic use (IVDR, CE marked under EU IVD regulation 2017/746) |
| COVID-19 R-GENE® | |
|---|---|
| PCR DESIGN | 2-PLEX
|
| TARGETED GENES |
|
| CONTROLS INCLUDED | Positive Control, Negative Control, Endogenous Internal Control |
| SPECIMENS |
|
| EXTRACTION PLATFORMS |
|
| AMPLIFICATION PLATFORMS |
|
| RESULT WITHIN | 90 minutes after extraction |
| STORAGE CONDITIONS | -15°C / -31°C |
| STATUS | For in vitro diagnostic use (IVDR, CE marked under EU IVD regulation 2017/746) |
| RHINO&EV/CC R-GENE® | |
|---|---|
| PCR DESIGN | 2-PLEX
|
| TARGETED GENES |
|
| CONTROLS INCLUDED | Positive Control, Negative Control, Endogenous Internal Control |
| SPECIMENS |
|
| EXTRACTION PLATFORMS |
|
| AMPLIFICATION PLATFORMS |
|
| RESULT WITHIN | 120 minutes after extraction |
| STORAGE CONDITIONS | -15°C / -31°C |
| STATUS | For in vitro diagnostic use (IVDR, CE marked under EU IVD regulation 2017/746) |