VIDAS® ToRC Panel
Serenity throughout Pregnancy
The VIDAS® ToRC panel includes 9 automated tests for the screening and diagnosis of toxoplasmosis, rubella, and cytomegalovirus (CMV) infections.
Disclaimer: Product availability varies by country. Please consult your local bioMérieux representative for product availability in your country.
- VIDAS ToRC Panel
- Overview
- Assays
- Resources
Overview
The VIDAS® ToRC panel is a complete solution for the diagnosis and monitoring of the most important materno-fetal infections in pregnant women and newborns. Toxoplasmosis, Rubella, and Cytomegalovirus (ToRC) infections are generally benign diseases. However when women are infected during pregnancy, consequences for the fetus can be dramatic. Our value-added IgG avidity tests help distinguish between recent and past infection, thus reducing the number of amniocenteses, and avoiding unnecessary treatment and stress for the future mothers.
A Complete Solution: 3 Markers for Accurate Diagnosis
- IgG: IgGs are widely used to determine the presence of specific immunity.
- IgM: If IgMs appear earlier than IgGs, they also disappear earlier. This makes IgMs an early and sensitive marker of acute infection.
- IgG avidity: IgGs are initially of low avidity but will mature to high avidity a few months after primary infection. A high IgG avidity index thus allows to rule out recently-acquired infection.
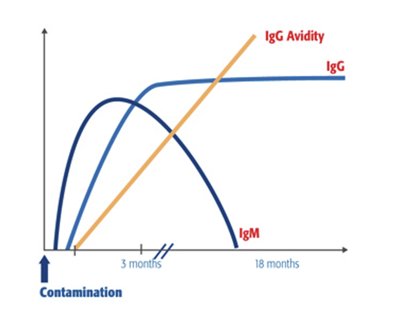
Kinetics of ToRC IgG and IgM antibodies and IgG avidity index
VIDAS® ToRC Solution
| Toxoplasmosis | Rubella | Cytomegalovirus |
|---|---|---|
| VIDAS® TOXO IgG II | VIDAS® RUB IgG | VIDAS® CMV IgG |
| VIDAS® TOXO IgM | VIDAS® RUB IgM | VIDAS® CMV IgM |
| VIDAS® TOXO Competition* | VIDAS® CMV IgG Avidity II | |
| VIDAS® TOXO IgG Avidity |
* TOXO Competition: screens for TOXO IgG/IgM (total antibody). Optimal test for areas of low prevalence.
Highly Performant IgG Avidity Tests
VIDAS® CMV IgG Avidity Tests
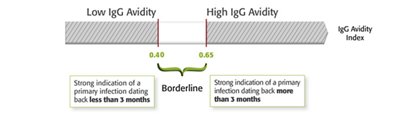
VIDAS® CMV IgG Avidity II is a simple technique which enables weak avidity antibodies to be differentiated from high avidity antibodies. The detection of high avidity antibodies is a strong indication of a primary infection of more than 3 months, whereas the detection of weak avidity antibodies is a strong indication of a primary infection of less than 3 months.
A study conducted to assess VIDAS® CMV IgG avidity II maturation kinetics on 135 sequential samples from 31 patients1 confirmed a very good correlation to clinical diagnosis:
- No misclassification of recent infections (< 3 months)
- No misclassification of past infections (> 3 months)
- Best correlation over time from onset of infection compared to other commonly used assays
- Most regular avidity maturation pattern
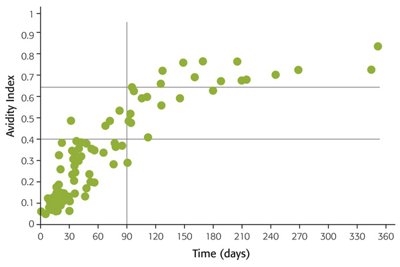
An accurate reflexion of the CMV–IgG avidity maturation kinetics
Adapted from Vauloup-Fellous C. et al.1
VIDAS® TOXO IgG Avidity
VIDAS® TOXO IgG Avidity is a simple technique which enables weak avidity antibodies to be differentiated from high avidity antibodies. The detection of high avidity antibodies is a strong indication of a primary infection dating back more than 4 months.
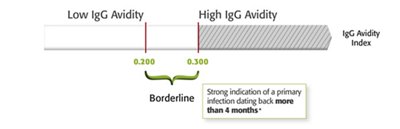
- Only FDA cleared Toxo IgG avidity test
- Best performance for the exclusion of primary infection in patients with IgM and IgG positive results compared to other avidity assays2,3
- The VIDAS® system holds a reference position in the field of toxoplasmosis serology
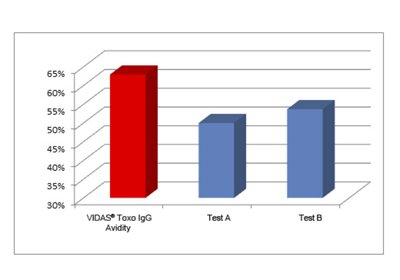
VIDAS® Toxo IgG Avidity identifies more old infections than other commonly used tests
See package inserts for performance details.
Ease of Use and Cost-Effectiveness
- Single-dose, ready-to-use tests
- Performed on the automated VIDAS® immunoanalyzers, recognized worldwide for their flexibility, ease-of-use, reliability, and 24/7 availability
- Small packaging volumes
- Ideal solution for small routine, confirmation of positive IgM, and IgG avidity testing
ToRC Testing During Pregnancy
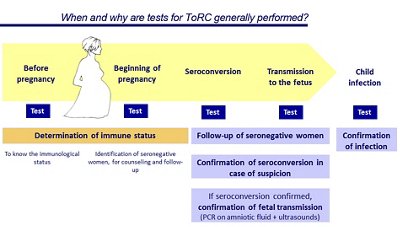
VIDAS® Solutions
- Reliable and easy-to-use instruments with random access and small footprint.
- Well adapted to rapid response laboratories.
- Factory-calibrated, Single-dose tests which reduce the need for additional controls.
- Short time to result.
References:
1. Vauloup-Fellous C.,et al. J Clin Virol (2012).
2. Villard O, et al. Clinical and Vaccine Immunology Journal. February 2013. | Evaluation performed by an independent network of experts.
3. Murat J-B., et al. Clinical and Vaccine Immunology Journal. August 2013.
Assays
Technical Specifications
| Assays | Reference | Code | Tests per Kit | Time to Result | Decisional Cut-offs or Measuring Range | |
|---|---|---|---|---|---|---|
| VIDAS® CMV IgG | 30 204 | CMVG | 60 | 40 minutes | 0 - 400 AU/mL | |
| VIDAS® CMV IgM | 30 205 | CMVM | 30 | 60 minutes | Qualitative test: Negative, Positive, or Equivocal | |
| VIDAS® CMV IgG Avidity II | 41 3557 | CMVA | 30 | 40 minutes | Avidity index (AI) An AI ≥ 0.65 is a strong indication of a primary infection dating back more than 3 months. An AI < than 0.40 is a strong indication of a primary infection dating back less than 3 months. An 0.40 < AI < 0.65 does not enable to distinguish a recent infection from a former infection. | |
| VIDAS® RUB IgG II | 30 221 | RBG | 60 | 40 minutes | 0 - 400 IU/mL | |
| VIDAS® RUB IgM | 30 214 | RBM | 30 | 60 minutes | Qualitative test: Negative, Positive, or Equivocal | |
| VIDAS® TOXO IgG II | 30 210 | TXG | 60 | 40 minutes | 0 - 300 IU/mL | |
| VIDAS® TOXO IgG Avidity | 30 222 | TXGA | 30 | 40 minutes | Avidity index (AI) An AI ≥ 0.3 is a strong indication of a primary infection dating back more than 4 months. An AI < than 0.3 does not enable a recent infection to be differentiated from a former infection. | |
| VIDAS® TOXO IgM | 30 202 | TXM | 60 | 40 minutes | Qualitative test: Negative, Positive, or Equivocal | |
| VIDAS® TOXO Competition | 30 211 | TXC | 60 | 40 minutes | Qualitative test: Negative, Positive |
BECAUSE IT MAKES SENSE ON VIDAS®

Resources
Related Publications
- Murat J-B., et al. Comparison of the Vidas System and Two Recent Fully Automated Assays for Diagnosis and Follow-Up of Toxoplasmosis in Pregnant Women and Newborns. Clinical and Vaccine Immunology Journal. August 2013.
- Villard O, et al. Comparison of four commercially available avidity tests for Toxoplasma gondii-specific IgG antibodies. Clinical and Vaccine Immunology Journal. February 2013.
- Vauloup-Fellous C., et al. Re-evaluation of the VIDAS® cytomegalovirus (CMV) IgG avidity assay : Determination of new cut-off values based on the study of kinetics of CMV-IgG maturation. J Clin Virol (2012).



